Pozycjonowanie stron www (SEO) 2024
Aktywny Model Rozliczeń
![]()
Co to jest pozycjonowanie stron?
Najprościej rzecz ujmując, odpowiedź na pytanie, co to jest pozycjonowanie strony, brzmi: to działania marketingowe, które mają na celu zdobycie i utrzymanie jak najwyższych pozycji dla określonych słów kluczowych w organicznych (bezpłatnych) wynikach wyszukiwania w wyszukiwarce internetowej (na przykład Google).
Pozycjonowanie strony obejmuje głównie działania związane z tworzeniem i optymalizacją treści w serwisie oraz pozyskiwaniem linków przychodzących, a ponadto optymalizacją techniczną witryny. Synergia tych działań pozwala na zwiększenie widoczności strony internetowej oraz jej lepsze dostosowanie do intencji użytkowników, co w efekcie powinno przełożyć się na osiąganie lepszych pozycji w wyszukiwarce, a tym samym zwiększenie ruchu w witrynie.
Na czym polega pozycjonowanie stron? W samym procesie pozycjonowania znaczenie mają zarówno kwestie techniczne, jak i te, które decydują o zadowoleniu użytkownika. Optymalnym kierunkiem jest więc takie pozycjonowanie stron, które przyniesie korzyści użytkownikom (poprzez wyświetlanie treści najlepiej pasujących do ich zapytań) oraz samej stronie, która znajdzie się wysoko w wynikach wyszukiwania (i dzięki temu pozyska ruch organiczny).
Korzystanie z wyszukiwarki w dalszym ciągu jest najpopularniejszym sposobem odkrywania i uzyskiwania treści online. Co więc skuteczne pozycjonowanie może zmienić w kontekście Twojego biznesu? Dobra strategia jest niezbędna dla poprawy ilości i jakości ruchu w Twojej witrynie. Warto jednak pójść o krok dalej i jeszcze przed rozpoczęciem pozycjonowania sprawdzić, jakie konkretnie korzyści ze sobą niesie.
Umów się na rozmowę.
Zmień podejście do SEO.
Wybierz AMR.
Pracuj ze zmotywowaną agencją!
Umów się na BEZPŁATNY BRIEF MARKETINGOWY. Zyskaj koncepcję skutecznego połączenia SEO z Twoim biznesem.
#wiemyjak
Jak działa pozycjonowanie stron? PORADNIK
Pozycjonowanie a SEO – czym się różnią?
Pozycjonowanie i SEO to pojęcia, które w Polsce często są używane zamiennie. Chcąc jednak być precyzyjnym, należy podkreślić, że występują między nimi istotne różnice. Choć oba odnoszą się do wyników wyszukiwania, w ich zakres wchodzą nieco inne działania.
Co to jest SEO? SEO (z ang. Search Engine Optimization) to inaczej optymalizacja stron pod kątem wyszukiwarek internetowych. Optymalizacja polega na poszukiwaniu najskuteczniejszego pod kątem pozycji danej witryny zestawu rozwiązań dotyczących treści, kodu strony, linków przychodzących oraz innych czynników wpływających na ranking w wyszukiwarce.
Optymalizacja strony obejmuje kilka różnych obszarów. Po pierwsze wprowadza się widoczne dla internautów zmiany w treści strony. Polegają one na odpowiednim rozmieszczeniu słów kluczowych tak, by strona wyświetlała się interesującym nas użytkownikom w wynikach wyszukiwania. Po drugie, działania związane z optymalizacją zakładają wprowadzenie zmian w kodzie witryny. Te co prawda nie mają bezpośredniego wpływu na wygląd strony, ale poprawiają jej parametry techniczne oraz użyteczność, usprawniając w ten sposób jej działanie. Ostatnią składową jest pozyskiwanie linków przychodzących, czyli wszystkich odnośników prowadzących do naszej strony z zewnętrznych źródeł.
Pozycjonowanie strony www jest z kolei procesem, na który składają się wszystkie działania (również wspomniana optymalizacja) umożliwiające danej stronie internetowej osiągnięcie jak najlepszej, czyli jak najwyższej i stabilnej, pozycji w wyszukiwarkach po wpisaniu konkretnej frazy. Pozycjonowanie jest ściśle powiązane z optymalizacją, dzięki której możliwe jest dostosowanie treści, linków oraz innych elementów strony internetowej tak, aby była ona jak najbardziej widoczna dla robotów wyszukiwarki. Zatem działania SEO, link building, content marketing i analityka internetowa wchodzą w skład pozycjonowania stron.
Jak więc można podsumować różnice między pozycjonowaniem strony internetowej a SEO? SEO zajmuje się udoskonalaniem serwisu – układem treści na stronie, budową kodu – oraz jego otoczenia, czyli linkami przychodzącymi. Na pozycjonowanie natomiast – poza wspomnianym już SEO – składa się również szereg innych działań. Być może traktowanie SEO i pozycjonowania jako synonimów wynika z faktu, że obu procesom przyświeca taki sam cel. W obu przypadkach chodzi o osiągnięcie wysokiej i stabilnej pozycji w wynikach wyszukiwania wyświetlanych przez wyszukiwarkę.
Na czym polega pozycjonowanie stron?
Pozycjonowanie stron internetowych nierzadko jest procesem długotrwałym, gdzie pierwsze wyniki mogą być widoczne nawet po kilku miesiącach. Nie jest przy tym działaniem tanim, jednak z biegiem lat firmy, które sukcesywnie inwestowały w skuteczne pozycjonowanie stron, dzisiaj zdobyły niewątpliwą przewagę nad tymi, które dopiero zaczynają interesować się tym tematem
Pozycjonowanie w internecie nie jest także jednolitym, powtarzalnym i uniwersalnym procesem gotowym do zastosowania w tej samej formie w każdej firmie niezależnie od branży, w jakiej funkcjonuje. Wręcz przeciwnie, o wiele ważniejsze jest indywidualne dopasowanie działań do potrzeb konkretnego biznesu i do konkretnej strony.
Więcej o tym, jak ocenić swoją witrynę w kontekście jej pozycjonowania w Google, pisaliśmy w artykule: Dobra strona internetowa, czyli jaka?
Wśród najważniejszych elementów, które należy wziąć pod uwagę, przygotowując wstępną strategię pozycjonowania, wymienić należy przede wszystkim branżę, w której działa klient, działania konkurencji oraz oczekiwania biznesowe. Od tych czynników będzie zależało, jakie konkretne działania w zakresie pozycjonowania stron powinny zostać wykonane na poszczególnych etapach. Natomiast etapy pozycjonowania najczęściej obejmują:
- Ustalenie celu i oczekiwań biznesowych klienta. Mają one kluczowe znaczenie podczas optymalnego doboru działań, które mogą pomóc w ich osiągnięciu. Ponadto określenie celów biznesowych daje również jasny obraz, do czego wspólnie dążą zarówno klient, jak i agencja SEO.
- Analiza obecnej sytuacji strony i wykonanie niezbędnych audytów. Pozycjonowanie stron firmy nie powinno być działaniem dla działania – przeprowadzane analizy powinny pomóc w ustaleniu strategii osiągnięcia jak najwyższych pozycji w wynikach wyszukiwania. Liczba obszarów do zbadania będzie zależała od sytuacji wyjściowej strony. Warto jednak przyjrzeć się przede wszystkim grupie odbiorców danej marki i ich potrzebom, co na przykład pomoże dobrać odpowiednie frazy kluczowe, a następnie przygotować zoptymalizowane pod nie treści. Ważnym obszarem do analizy są także strony konkurencji – między innymi w zakresie publikowanych treści, wprowadzonych na stronach www rozwiązań czy innych działań. Audyt SEO pozwala także zorientować się, jakie problemy występują w witrynie i które obszary stopują sukces w wynikach wyszukiwania.
- Planowanie i przygotowanie strategii pozycjonowania stron. Strategia SEO to długoterminowy plan działania – a w czasach, gdzie konkurencja w internecie jest coraz większa, pozycjonowanie stron www powinno być niezbędnym elementem strategii marketingowej każdej firmy.
- Wdrożenie rekomendacji. Mogą to być rekomendacje dotyczące optymalizacji technicznej, nowa struktura strony czy publikowanie wartościowych treści. Niektóre wdrożenia mogą odbyć się już na początkowym etapie pozycjonowania, inne natomiast będą rozłożone w czasie.
- Monitorowanie wyników. Przydatne do mierzenia wyników będą takie narzędzia jak Google Search Console, Google Analytics czy Senuto. Przy współpracy z agencją oczywiście dodatkowo pojawią się raporty z przeprowadzonych działań. Regularne monitorowanie efektów prowadzonych działań umożliwia weryfikację, czy obrana strategia realizuje założony biznesowy cel.
Z punktu widzenia właścicieli firm, skuteczne pozycjonowanie strony internetowej w Google to inwestycja, która pozwala czerpać korzyści z większego zasięgu i rozpoznawalności marki. Korzystając z profesjonalnej pomocy agencji SEO możesz skupić się przede wszystkim na swoim biznesie.
Co wpływa na pozycjonowanie strony?
O miejscu zajmowanym przez serwis w wynikach wyszukiwania decyduje algorytm Google. Na jakiej podstawie ocenia on jednak witryny i co wpływa na pozycjonowanie strony www?
Szczegółowy sposób działania algorytmu nie jest znany. Ponadto algorytmy Google są regularnie aktualizowane, a wprowadzane zmiany znajdują odzwierciedlenie w wyświetlanych wynikach. Znane są jednak pewne uwarunkowania, czyli czynniki rankingowe Google, które mają wpływ na to, że dana strona ma szansę znaleźć się wyżej w wynikach wyszukiwania. Liczba tych czynników jest ogromna, a dodatkowo nie wszystkie są jawne. Śledząc wiadomości od Google oraz z branży SEO o tym, co wpływa na pozycjonowanie strony w internecie, można zadbać o swoją stronę internetową, niemniej warto, aby usługa pozycjonowania była realizowana przez profesjonalistów.
Czynniki, które wpływają na algorytmy wyszukiwarek, cały czas ewoluują, żeby nadążyć za zmieniającymi się zachowaniami użytkowników i postępami w uczeniu maszynowym. Warto jednak przyjrzeć się tym podstawowym, aby zrozumieć, co jest niezbędne, aby zoptymalizować strony pod kątem pozycji w Google.
Wśród czynników, które mają wpływ na pozycjonowanie www, możemy wyróżnić:
- czynniki związane z treścią na stronie – ilość treści na stronie, ich jakość i unikalność, występowanie słów kluczowych, częstotliwość dodawania treści, optymalizacja grafik, zdjęć, filmów i innych elementów graficznych, Core Web Vitals;
- czynniki związane z profilem linkowym strony – linkowanie zewnętrzne, czyli liczba linków z innych stron, ich jakość oraz linkowanie wewnętrzne;
- czynniki związane z optymalizacją techniczną – prędkość ładowania strony, mapa witryny;
- czynniki związane z domeną – historia domeny, kto jest/był właścicielem domeny.
Czym jest pozycjonowanie on-site?
Pozycjonowanie on-site koncentruje się na ulepszeniu i optymalizacji elementów w obrębie witryny internetowej. Są to więc wszystkie procesy z zakresu pozycjonowania, które są bezpośrednio związane ze stroną, z jej użytkowaniem, dostępnością i atrakcyjnością dla użytkownika, a także widocznością dla wyszukiwarek.
Elementy w obrębie witryny, czyli treści (teksty, grafiki, wideo, zdjęcia) powinny być zoptymalizowane zarówno pod użytkowników, jak i wyszukiwarki. To właśnie te dwie grupy są głównymi odbiorcami Twojej witryny. Optymalizacja konieczna jest także w przypadku kodu.
Optymalizacja treści jest jednym z najważniejszych obszarów działań pozycjonowania on-site. Teksty publikowane w serwisie muszą zawierać odpowiednio dobrane dla danej podstrony słowa kluczowe, a przy tym powinny być aktualne, merytoryczne, wysokiej jakości, bez błędów gramatycznych czy ortograficznych oraz czytelne.
Dla wyszukiwarek istotna będzie optymalizacja meta tagów, adresów url, nagłówków (H1-H6) oraz tekstów alternatywnych (altów). Meta dane, a zwłaszcza meta title i meta description, mają też znaczenie dla użytkowników. Jako że są one widoczne w wynikach wyszukiwania, mogą decydować, czy użytkownik kliknie w link właśnie do Twojej strony. Mogą więc być dodatkowym narzędziem służącym do przyciągnięcia uwagi użytkownika.
Czym jest pozycjonowanie off-site?
Podstawową różnicą między pozycjonowaniem on-site a pozycjonowaniem off-site jest to, że w drugim przypadku widoczność serwisu jest budowana za pomocą innych kanałów niż Twoja własna strona. Są to zatem wszystkie działania, które wykonujesz poza własną witryną.
Działaniem najbardziej kojarzonym z SEO off-site jest link building, czyli pozyskiwanie linków prowadzących do Twojej strony z odpowiednich, wiarygodnych i zaufanych witryn. Warto również pamiętać, że jakość tych linków jest ważniejsza niż ich liczba. Celem jest więc pozyskanie nie tylko dużej liczby linków, ale przede wszystkim powinny one być wysokiej jakości.
SEO off-site to również inne działania wspierające promocję witryny w internecie. Można do nich zaliczyć:
- marketing, PR i budowanie marki – wszystkie działania, które mają na celu zwiększenie rozpoznawalności i reputacji marki, a także współpracę z mediami (co również może wspierać pozyskiwanie linków zewnętrznych prowadzących do naszej strony internetowej);
- gościnne wpisy na blogach lub inne formy marketingu treści w zewnętrznych źródłach;
- social media marketing – udostępnianie wartościowych treści swoim odbiorcom to także sposób na zdobywanie linków, ale przede wszystkim działania w social mediach pomagają w promocji marki, interakcji z klientami i zachęcaniu do skorzystania z oferty;
- oceny i recenzje – zdobywanie nowych, monitorowanie i reagowanie na wzmianki pojawiające się o marce. Pomocna może być tutaj Wizytówka Google, która również pozwala na budowanie relacji z potencjalnym klientem.
Na czym polega optymalizacja techniczna w procesie pozycjonowania?
Techniczne SEO jest częścią procesu pozycjonowania i polega na poprawie technicznych aspektów strony internetowej, co ma prowadzić do zwiększenia jej widoczności w wynikach wyszukiwania. Najważniejszym celem skutecznej optymalizacji technicznej jest sprawienie, by strona była szybsza, łatwiejsza do zaindeksowania i bardziej zrozumiała dla wyszukiwarek.
Założeniem Google i innych wyszukiwarek jest przedstawienie użytkownikom wyników możliwie najlepiej dopasowanych do ich zapytań. Z tego powodu roboty Google indeksują i oceniają strony internetowe na podstawie wielu czynników. Część z nich odnosi się do technicznych aspektów strony. Przykładowo, optymalizując dane strukturalne, pomagasz robotom wyszukiwarek zrozumieć, o czym jest Twoja strona. Dzięki temu są w stanie właściwie zinterpretować jej zawartość i wyświetlać jako wynik wyszukiwania na konkretne zapytania użytkowników.
Proces optymalizacji technicznej rozpoczyna się od przeprowadzenia audytu, co pozwala na identyfikację kluczowych problemów i wskazanie aspektów wymagających poprawy. Na podstawie audytu powstaje plan działań w zakresie optymalizacji technicznej, która odnosi się między innymi do takich elementów jak architektura witryny, dostosowanie do użytkowników mobilnych czy szybkość strony.
Dla ułatwienia wyszukiwarkom odkrywania i uzyskania dostępu do zawartości Twojej strony znaczenie będą miały takie elementy techniczne jak: struktura adresu URL, nawigacja na stronie czy linkowanie wewnętrzne. Elementy techniczne wpływają również na doświadczenie użytkownika związane z korzystaniem z serwisu – są to prędkość ładowania, dostosowanie strony do urządzeń mobilnych czy bezpieczeństwo witryny.
Dobrze zoptymalizowana pod kątem technicznym strona internetowa to taka, która:
- odpowiednio szybko się ładuje,
- może zostać zaindeksowana przez wyszukiwarki,
- ma jedną wersję dostępną dla użytkowników i robotów indeksujących,
- ma ważny certyfikat SSL zapewniający bezpieczeństwo korzystania z serwisu,
- ma uporządkowane dane strukturalne,
- ma mapę witryny xml,
- jest dostosowana do urządzeń mobilnych,
- w przypadku stron mających różne wersje językowe – zawiera atrybuty hreflang.
Czy pozycjonowanie strony internetowej jest skuteczne
Podczas pozycjonowania strony internetowej najważniejszym celem jest uzyskanie jak najwyższego miejsca pozycjonowanego serwisu w wynikach wyszukiwania, wyświetlanych po wpisaniu przez użytkowników do wyszukiwarki interesujących nas haseł. Najpopularniejsze wyszukiwarki, w tym dominująca na rynku – Google, wyświetlają przy standardowych ustawieniach zazwyczaj 10 odnośników do stron (pomijając reklamy i różnego rodzaju dodatkowe funkcje wyszukiwania).
Ze względu na to, że około 90% użytkowników korzysta tylko z pierwszej strony wyników i nie szuka informacji ukrytych dalej, tzw. TOP 10 uważa się powszechnie za podstawowy cel pozycjonowania stron.
Liczba wyszukiwań nieustannie rośnie – poniżej przedstawiamy dane dotyczące ilości wyszukiwań na przestrzeni lat.

Jak wygląda pierwsza strona w wynikach wyszukiwania?
Wpisując w wyszukiwarce dowolne hasło, zobaczysz pierwszą stronę wyników wyszukiwania, która może prezentować się w taki sposób:

Wygląd pierwszej strony wyników wyszukiwania może nieznacznie zmieniać się w zależności od wyszukiwanego hasła – w tym przypadku na górze widać płatną reklamę produktową, czyli Google Shopping. Następne wyniki to również płatne reklamy tekstowe, czyli linki sponsorowane. Poniżej widoczne są naturalne wyniki wyszukiwania, a po prawej stronie tzw. graf wiedzy (pokazujący dodatkowe informacje o szukanym obiekcie).
Pozycja w wynikach wyszukiwania a klikalność
W tabeli poniżej znajdziesz informacje, jak duży wpływ ma osiągana przez Twoją stronę pozycja w rankingu wyników wyszukań Google na klikalność, czyli stosunek liczby kliknięć w link do liczby wyświetleń (na komputerach stacjonarnych – desktop i w urządzeniach mobilnych – mobile). Dane zostały udostępnione przez Advanced Web Ranking i pochodzą z maja b.r. – pokazują określony współczynnik klikalności CTR (%) dla danych pozycji. Co to oznacza? Jeśli Twoja strona znajdzie się na pierwszej pozycji w wynikach wyszukiwań, średnio 28,93% użytkowników, którzy zobaczą te wyniki, kliknie w Twój link.

Pozycjonowanie strony internetowej a algorytm Google
Znając już dane na temat ilości użytkowników oraz klikalności poszczególnych wyników, wiemy, jak olbrzymi zasięg ma wyszukiwarka i jak ważne jest, by być na pierwszej stronie wyników, a co za tym idzie – dlaczego warto inwestować w pozycjonowanie. Pojawia się jeszcze pytanie – jak to robić i na jakie czynniki należy zwracać uwagę?
Kolejność, w jakiej pojawiają się konkretne podstrony, nie jest przypadkowa – ranking w wyszukiwarce jest ustalany na podstawie ponad 200 mierzalnych i jednoznacznych czynników, a za jego ułożenie odpowiada algorytm wyszukiwarki. Oznacza to, że pod uwagę brane są tylko te elementy, które bez cienia wątpliwości można przypisać do danego adresu www w internecie. Ważne jest także, że poszczególne czynniki mogą mieć zarówno pozytywny, jak i negatywny wpływ na wyniki.
Nie jest znana pełna lista czynników, które Google bierze pod uwagę, ale od marca 2018 r. wiemy, że najważniejsze są 3 elementy*:
- content na stronie i linki prowadzące do serwisu,
- RankBrain – system uczący się Google, który, jako część algorytmu, interpretuje zapytania wprowadzane do wyszukiwarki i pomaga w wyświetlaniu wyników zgodnych z intencjami użytkowników, niekoniecznie poprzez ścisłe dopasowanie słów kluczowych tak, jak to się działo wcześniej.
Pozycjonowanie, które pomoże Ci zdobyć pierwsze pozycje
Wyścig o pierwsze pozycje dla Twojej strony w wyszukiwarce to złożony proces, uwzględniający działania z zakresu optymalizacji technicznej, rozbudowy strony pod kątem treści i pozyskiwania wartościowych linków – proces ten wymaga czasu oraz sporego wysiłku. Punktem wyjścia dla naszych działań jest to, ile pracy do chwili obecnej wykonała konkurencja przy pozycjonowaniu swoich stron na daną frazę.
Istnieją branże, w których niezwykle trudno będzie się przebić, bo witryny obecne w TOP 10 mają za sobą wiele lat pozycjonowania oraz setki tysięcy złotych wydane na wyprzedzenie konkurencji i utrzymywanie swoich wyników. Wysokie pozycje dla firm bardziej niszowych, gdzie konkurencja nie jest tak silna, będą zdecydowanie łatwiejsze do osiągnięcia, ale też – jak łatwo się domyślić –wiązać się to będzie z mniejszym ruchem oraz niższą konwersją.
Jak widać, pozycjonowanie i SEO musi wiązać się z szeregiem działań z zakresu optymalizacji Twojej strony (poprawa kodu i struktury), tworzenia i pracy z treściami (rozbudowa Twojej strony) oraz link buildingu (pozyskiwanie linków prowadzących do Twojej strony). Przy realizacji strategii pozycjonowania Twojej strony zwracamy też – zgodnie z zaleceniami Google – szczególną uwagę również na content marketing, czyli tworzenie i dystrybucję użytecznych oraz wartościowych treści dla Twoich potencjalnych klientów oraz analitykę internetową, która pozwala nam zmierzyć efekty prowadzonych działań i zwiększyć ich skuteczność.
Efektywne pozycjonowanie to wzrost ruchu na Twojej stronie
Ze względu na ogromną liczbę użytkowników w wyszukiwarkach ruch z wyników organicznych jest zazwyczaj najważniejszym źródłem ruchu na Twojej stronie internetowej. W procesie pozycjonowania ważne jest, aby mierzyć siły na zamiary i przy założonym budżecie na działania uzyskiwać odpowiedni zwrot z inwestycji.
Można przyjąć kilka taktyk pozyskiwania ruchu na stronę www. Wydawać się może, że najskuteczniejszą metodą na zdobycie dużego ruchu będzie wypozycjonowanie serwisu na najmocniejsze frazy kluczowe, definiujące daną branżę. Stosowanie takiego podejścia może jednak skutkować tym, że przez długi czas nie zauważysz efektów – aż do momentu osiągnięcia zamierzonych pozycji. Zaczynając od łatwiejszych słów i stopniowo zwiększając ich liczbę, masz szansę dość szybko odczuć wzrosty ruchu – początkowo niewielkie, stopniowo powiększające ilość użytkowników na Twojej stronie.
Spójrzmy zatem, na przykładzie danych jednego z naszych klientów, jak zwiększająca się widoczność wpłynęła na ruch w kontekście wyników organicznych oraz jakie miało to przełożenie na cały ruch na stronie:

Niezależnie więc od tego, czy po raz pierwszy słyszysz o pozycjonowaniu stron www, czy też Twoja strona była już widoczna na w wynikach wyszukiwania Google, musisz wiedzieć, że widoczność GWARANTUJE wzrost ruchu i daje szansę na wysoką sprzedaż.
Kiedy warto zdecydować się na pozycjonowanie?
Zamów ofertę na pozycjonowanie strony www, jeśli:
- Twoje produkty/usługi już się sprzedają (np. online, dzięki kampaniom Google Ads lub offline),
- chcesz rozwijać swój biznes,
- strona internetowa jest już Twoim głównym kanałem marketingu i sprzedaży,
- dopiero chcesz zbudować internetowy kanał dystrybucji,
- chcesz sukcesywnie zwiększać ruch na swojej stronie,
- oferujesz produkty i usługi, których klienci szukają w Google,
- wiesz, że musisz być widoczny w wynikach wyszukiwania mimo tego, że sprzedajesz tylko offline,
- chcesz zwiększyć sprzedaż,
- myślisz strategicznie i długoterminowo,
- chcesz budować markę online i offline.
Pozycjonowanie krok po kroku
Wiesz już, jak dobrać odpowiednie dla Twojego biznesu słowa kluczowe. Czas więc, żebyś dowiedział się więcej o innych krokach podejmowanych w procesie pozycjonowania stron www. To ta część pracy, którą zajmujemy się na co dzień.
Przygotowanie elementów do optymalizacji
Proces pozycjonowania jest bardzo złożony, a jednym z ważniejszych zadań jest wybranie i przygotowanie elementów do jej optymalizacji. W podjęciu odpowiedniej decyzji pomogą nam wnioski z analizy i audytu witryny, warto też sugerować się wskazówkami jakościowymi Google. W pierwszej kolejności możemy zająć się optymalizacją na przykład zweryfikowanej struktury i nawigacji witryny, zmierzonego czasu ładowania strony internetowej czy przeanalizowanej treści na serwisie (włącznie z metatagami i strukturą nagłówków).
Wdrożenie zmian w ramach optymalizacji SEO
Odpowiednio przygotowana optymalizacja serwisu przewiduje wprowadzenie wielu modyfikacji – najczęściej w profilu linkowym, treściach i kodzie źródłowym strony. Bardziej szczegółowo wymienić możemy między innymi wprowadzenie zoptymalizowanych pod kątem fraz kluczowych opisów na poszczególne podstrony serwisu, wdrożenie niezbędnych zmian w indeksie strony, konstrukcji adresów URL i strukturze przekierowań czy też naprawę błędów walidacji W3C. Najważniejsze jednak, że za modyfikację strony odpowiadają wykwalifikowani Web-Developerzy, a nad całym procesem czuwa opiekun SEO Twojego serwisu.
Tworzenie skutecznych i wartościowych treści
Pozycjonowanie sklepów internetowych czy serwisów usługowych nie przyniesie oczekiwanych rezultatów bez rozbudowy istniejących i tworzenia nowych treści zoptymalizowanych pod odpowiednie dobrane frazy kluczowe. Dzięki temu w sposób efektywny, a jednocześnie naturalny, dodajemy do serwisu słowa kluczowe. Nowe teksty będą też stanowić wartość dodaną dla osób odwiedzających Twoją stronę, które będą mogły zapoznać się z rzetelnie przygotowanymi opisami produktów, kategorii czy artykułami poradnikowymi.
Analiza wyników i dalsza optymalizacja strony internetowej
Po wdrożeniu niezbędnych poprawek nasza praca się nie kończy, bo stale obserwujemy zmiany w widoczności Twojej strony w naturalnych wynikach wyszukiwania. Dzięki temu możemy na bieżąco analizować, jakie efekty przynoszą nasze działania, ale również szybciej zareagujemy, gdy zmiany – zgodnie z założeniem, że testować należy różne rozwiązania – nie będą skuteczne, a sama strategia pozycjonowania strony będzie wymagała korekty, aby było możliwe osiągnięcie lepszych pozycji w wynikach wyszukiwania oraz większego ruchu na stronie.
Ile trwa pozycjonowanie strony?
Pozycjonowanie sklepu internetowego czy innej strony internetowej jest procesem, który zabiera sporo czasu.
Chcąc podnieść pozycję witryny w wynikach wyszukiwania, trzeba nie tylko posiadać ciekawą ofertę asortymentową, która przemawiać będzie do konkretnego klienta. Konieczne jest także podjęcie działań, takich jak optymalizacja i pozycjonowanie.
Nie powinieneś mieć jednak złudzeń, że w krótkim czasie można osiągnąć wysoką pozycję w rankingu wyszukiwarki Google. Warto znać podstawy pozycjonowania i wiedzieć ile trwa pozycjonowanie i po jakim czasie można spodziewać się pierwszych efektów działań SEO (Search Engine Optimization) w wynikach wyszukiwania?
Jeśli dopiero poznajesz podstawy pozycjonowania www, powinieneś poczytać trochę o sposobie oceny witryn stosowanym przez Google. Roboty działające w wyszukiwarce pełzają po stronach internetowych i oceniają ich treść, jakość, ilość wystąpień słów kluczowych czy linki wychodzące. Jeśli Google zauważy, że wciąż modyfikujesz stronę, starasz się ją udoskonalać, poprawiać, stosując przy tym zasady dobrego SEO, wyszukiwarka doceni Twoje działania. Roboty będą częściej odwiedzały witrynę, dzięki czemu będzie ona częściej indeksowana. W konsekwencji będziesz miał możliwość uzyskania lepszej pozycji w rankingu. Przyjęcie podejścia procesowego w pozycjonowaniu stron www, czyli podejmowanie systematycznych działań SEO, przyczyni się do osiągnięcia wysokiej i stabilnej lokaty w wynikach wyszukiwania.
Kiedy zobaczysz efekty pozycjonowania?
To, kiedy zobaczysz pierwsze efekty pozycjonowania, jest uzależnione od kilku czynników, a przede wszystkim:

Choć wiesz już, że Google docenia modyfikacje strony internetowej, zastanawiasz się pewnie, ile trwa pozycjonowanie stron internetowych. Nie można udzielić jednoznacznej odpowiedzi na to pytanie, gdyż podjęcie takich samych działań SEO nie będzie tak samo efektywne w przypadku różnych stron internetowych. Pozycjonowanie stron internetowych w Google zależy głównie od budżetu na działa SEO oraz przyjętej strategii SEO. Długość okresu, po którym wystąpią pierwsze efekty pozycjonowania, zależy między innymi od:
- branży, w której działasz – w jednej branży łatwiej jest zaistnieć, w innej potrzeba dużo więcej czasu, warto tutaj też uwzględnić to, czy działasz w całym kraju, czy tylko w swoim regionie;
- profesjonalisty od pozycjonowania, z którego usług korzystasz – dobry specjalista, nie zapewni Ci pozycji w miesiąc, ale będzie podejmował rozsądne działania tak, aby w możliwie najkrótszym czasie Twoja pozycja nie tylko stała się wysoka, ale także stabilna w wynikach wyszukiwania;
- zastosowanej strategii SEO – nie każdy pozycjoner ma taką samą strategię, dla jednych ważny jest szybki wzrost pozycji oraz wzrost ruchu na stronie www, nie patrząc przy tym na konsekwencje, inni zdecydują się na kompleksowe pozycjonowanie stron, które zajmuje więcej czasu, ale przynosi większe korzyści;
- historii prowadzonej przez Ciebie strony – jeśli strona ma nieciekawą historię, czyli została przez Google uznana za łamiącą zasady, najpierw należny odbudować do niej zaufanie, a to wydłuża czas oczekiwania na efekty;
- znajomości podstaw pozycjonowania – dzięki temu będziesz wiedział, na co przeznaczasz pieniądze i specjalista SEO nie będzie musiał Cię przekonywać, że dany wydatek jest uzasadniony, przez co szybciej zostaną wdrożone niezbędna działania;
- intensywności działań konkurencji – jeśli Twoi konkurenci również poświęcają dużo czasu i energii, aby zbudować widoczność w wyszukiwarce, musisz liczyć się z tym, że by ich pokonać, będziesz potrzebował czasu i wytrwałości w działaniach.
Pozycjonowanie stron w Google – gdzie warto być?
Pozycjonowanie strony to nie tylko standardowe działania w wyszukiwarce – obecnie masz zdecydowanie więcej możliwości, by zwiększyć widoczność swojej strony w sieci. Gdzie warto być?
Google News
Przede wszystkim – jeśli np. posiadasz bloga lub portal informacyjny – możesz skorzystać z publikacji tworzonych w nim artykułów w Google News. To agregator gromadzący opinie, analizy i artykuły dotyczące m.in. aktualnych wydarzeń, który – gdy te odpowiadają na wprowadzone w Google zapytanie – wyświetla je na pierwszej stronie wyników wyszukiwania. Co to oznacza? Wysoką pozycję strony. bez nadmiernego nakładu pracy. Żeby jednak wszystko to było możliwe, Twoja strona powinna spełnić szereg wymagań technicznych oraz formalnych. Po podłączeniu jej do Google News pamiętaj, aby każda prezentowana przez Ciebie treść zawierała ważne i wartościowe – z punktu widzenia użytkownika – informacje. Jednocześnie wiadomości muszą być aktualne, bo tylko takie zostaną przez Google zaakceptowane. „Wiadomości” to nie miejsce dla felietonów, instrukcji czy porad, lecz dla treści przyciągających uwagę i opisujących zdarzenia rzeczywiste.
Google Grafika
Cała sieć jest obecnie wypełniona fotografiami uzupełniającymi publikowane treści, co oznacza, że i na Twojej profesjonalnej stronie zdjęcia na pewno się pojawią. Jest to świetna okazja, by wykorzystać ich potencjał w skutecznym pozycjonowaniu stron www w Grafikach Google – to kolejne źródło, w którym użytkownicy poszukują kluczowych dla nich informacji. Jeśli chcesz odnieść sukces na tym polu i w ten sposób przyciągnąć ruch na swoją stronę internetową, musisz pamiętać o kilku parametrach, które mogą mieć na to wpływ:
- Parametr ALT – zawsze uzupełniaj alternatywny tekst (słowa kluczowe), który – w przypadku problemów wyszukiwarki z wczytywaniem zdjęcia – będzie się wyświetlał w jego miejscu. Opis ALT powinien być unikalny i zrozumiały, bo jego główną rolą jest poinformować użytkownika, co znajduje się na zamieszczonym na stronie zdjęciu.
- Linki prowadzące do obrazka – pojawiają się po to, by zarówno użytkownik, jak i roboty Google mogły z łatwością do niego dotrzeć. Ważne, żeby link do grafiki znajdował się na kilku podstronach serwisu, będzie to czytelna informacja dla robotów, że obrazek jest ważny.
- Nazwa pliku ze zdjęciem – powinna być związana z tym, co przedstawia grafika, jak i również słowami kluczowymi, które chcemy w ten sposób wypozycjonować.
Warto też zadbać o tytuł grafiki i treść, którą ją otacza, tytuł strony, na której grafika się znajduje i lokalizację obrazka na serwerze, wagę oraz rozmiar zdjęcia, a także jego odpowiedni format.
Efekty pozycjonowania stron i ich grafik znajdziesz w Google Search Console w dziale skuteczność.
YouTube
YouTube to potężny serwis internetowy, na którym bezpłatnie można umieszczać, odtwarzać, oceniać i komentować filmy. To jednocześnie kolejne miejsce, w którym przebywa niezliczone grono potencjalnych klientów, jeszcze nieświadomych, że są zainteresowani Twoimi usługami. I tutaj warto więc się pozycjonować. Jak to robić najlepiej? Musimy pamiętać o kilku zasadach:
- Zadbaj o odpowiedni tytuł, który nie tylko zachęci użytkowników do obejrzenia Twojego filmu, ale będzie także zawierał właściwe słowa kluczowe.
- Przygotuj miniaturę, która przykuje uwagę i sprawi, że użytkownicy będą chcieli sprawdzić, co dla nich przygotowałeś.
- Dobierz użyteczny opis – informujący o tym, co znalazło się w Twoim filmie i zoptymalizowany pod kątem fraz kluczowych, a także pasujący stylem do tytułu i miniatury. Pamiętaj koniecznie, by umieścić w nim link do Twojej strony firmowej. Zastanów się również nad linkami do najciekawszych fragmentów filmu i ewentualnych materiałów dodatkowych.
- Stwórz tematyczne SEO tagi, które pozwolą z łatwością odnaleźć Twój film wśród konkurencji.
- Zwróć uwagę na nazwę pliku (bez polskich znaków!) – ona również może zawierać w sobie pomocne słowa kluczowe.
- Bądź dostępny – odpowiadaj na komentarze użytkowników – przy odrobinie Twojego zaangażowania i pomocy ze strony SEO oni mogą stać się Twoimi klientami!
Efekty pozycjonowania stron, a tak naprawdę ich filmów weryfikujemy w panelu YouTube, tam gdzie filmy zostają przesyłane.
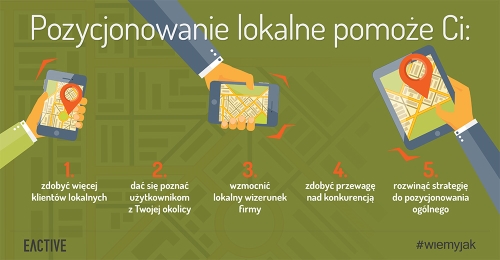
Google Maps / Google Business
Kluczowym miejscem, w którym powinieneś prowadzić działania z zakresu pozycjonowania Twoich stron, są też Mapy Google. Mechanizm wyświetlania wyników dopasowanych do obecnego położenia użytkownika wyszukiwarki Google (wykorzystanie geolokalizacji) najskuteczniej wykorzystywany jest w pozycjonowaniu lokalnym. Na czym to polega? Posłużmy się przykładem. Załóżmy, że mieszkasz na stałe we Wrocławiu, w łazience zepsuł Ci się kran, więc potrzebujesz pomocy fachowcy – hydraulika – który naprawi tę niespodziewaną usterkę, ewentualnie sprawdzi też instalację, a może nawet zakupi nową baterię łazienkową i od razu ją wymieni. Wpisując w wyszukiwarce hasło „hydraulik”, oczekujesz, że w wynikach pojawią się firmy z Twojej okolicy, a nie z drugiego krańca Polski – szukasz pomocy, która będzie „pod ręką”. Google doskonale o tym wie, dlatego – mając możliwość określenia Twojej obecnej lokalizacji za pomocą GPS – w odpowiedzi na Twoje zapytanie wyświetli przede wszystkim mapkę z pobliskimi punktami usług hydraulicznych wraz z podstawowymi informacjami na ich temat. Na przykład zapytanie z lokalizacji Wrocław, wyświetli wyniki z Wrocławia nawet gdy użytkownik nie dopisze miasta – w tym wypadku stolicy Dolnego Śląska.
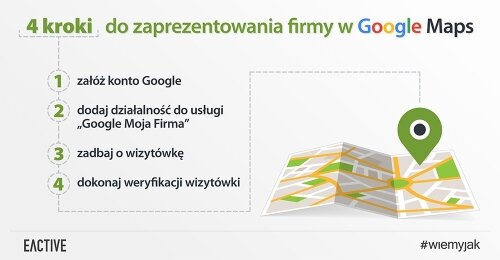
Co zrobić, by Twoja firma mogła znaleźć się w tych wynikach? Zacznijmy od podstaw. Po utworzeniu konta w Google (jeśli dotychczas go nie zakładałeś), zarejestruj się w Google Moja Firma i stwórz tam profil własnej działalności, pamiętając o opublikowaniu wszystkich istotnych dla użytkowników informacji. Poza tymi oczywistymi, jak nazwa firmy, dane kontaktowe, godziny otwarcia, czy oferta produktowa, zatroszcz się też o unikalny opis swojej firmy (pamiętaj przy tej okazji o frazach kluczowych) oraz atrakcyjne zdjęcia i grafiki (również odpowiednio opisane). Swoją wizytówkę koniecznie połącz z firmową stroną internetową – umieszczając w Mapach Google link do Twojej strony www, to samo zrób na stronie, publikując odnośnik do wizytówki w Google.
Na pozycjonowanie stron internetowych w Mapach Google poprzez wizytówkę Google Moja Firma wpływają też inne działania, takie jak pozytywne opinie użytkowników (warto zadbać o to, aby Twoi Klienci zostawiali sporo takich pozytywnych opinii – Google promuje firmy z najwyższą średnią ocen) oraz regularna publikacja wpisów. Wpisy w Google dla firm działających lokalnie to stosunkowo nowa funkcjonalność, o której nie wszyscy jeszcze wiedzą, czyli szansa na wyprzedzenie Twojej konkurencji w wyszukiwarce. Pozwoli Ci ona na umieszczanie ogłoszeń i ofert, informowanie swoich potencjalnych Klientów o dostępności nowych lub popularnych produktów oraz o wydarzeniach, które organizujesz w swoim sklepie.
Ile kosztuje pozycjonowanie stron?
Pozycjonowanie stron wyceniamy zawsze w taki sposób, byśmy mieli realną szansę na spełnienie naszej obietnicy, czyli takie wykonanie usług pozycjonowania stron internetowych, które w znaczący sposób zwiększy Twoją widoczność w Google. Bez dobrze obliczonego budżetu już na starcie jesteśmy skazani na porażkę. Brak odpowiednich środków powoduje, że nie jesteśmy w stanie prowadzić takiej ilości działań marketingowych, które pozwolą nam nadążyć za rynkiem oraz za konkurencją.
Od czego zależy cennik pozycjonowania?

Pamiętaj, że:
- cena za pozycjonowanie zależy od wybranych fraz kluczowych – im słowa kluczowe będą bardziej konkurencyjne, tym cena będzie wyższa,
- pozycjonujemy nielimitowaną ilość słów kluczowych,
- pozycjonujemy całe strony internetowe w Google, a nie kilka wybranych słów kluczowych,
- koncentrujemy się na realizacji Twoich celów biznesowych, najczęściej jest to znaczący wzrost sprzedaży oraz jej rentowności a także wzrost ilości i jakości leadów,
- nasze wyceny zaczynają się od 8000 zł,
- rozliczamy się w AKTYWNYM MODELU zorientowanym na Twoje cele (Abonament to przestarzała i wysoce nieskuteczna metoda),
- oferujemy umowę na czas nieokreślony.
Skuteczne pozycjonowanie stron to szansa dla Twojego biznesu
Nasze doświadczenie pokazuje jednak, że widoczność w internecie to obecnie podstawa dla wielu przedsiębiorców, którzy chcą docierać do nowych klientów i rozwijać swój biznes. Pojawienie się Twojej strony na wysokich pozycjach w wyszukiwarce Google daje gwarancję zwiększenia na niej ruchu i ilości użytkowników, a w efekcie – wpływa na wzrost sprzedaży. Nie możesz jednak zapominać, że pozycjonowanie to proces długotrwały i najlepsze rezultaty będą widoczne dopiero po dłuższym czasie.
Pozycjonowanie w wynikach wyszukiwania zwiększy widoczność strony w Google w wynikach wyszukiwania, a co za tym idzie nastąpi podniesienie ruchu na stronach www.
Widoczność w wynikach wyszukiwania strony w Google pozwoli Ci wzbudzić zaufanie i zbudować świadomość swojej marki. Pojawianie się Twojego serwisu w wynikach wyszukiwania pod wieloma frazami kluczowymi sprawi, że Twoi potencjalni klienci zaczną Cię kojarzyć. Wysokie pozycje zapewniają też wiarygodność w oczach użytkowników, bo z reguły ufają oni Google i klikają w linki, które wyświetlają im się jako pierwsze w wyszukiwarce.
Google jest obecnie najpopularniejszą wyszukiwarką wśród polskich użytkowników. Ilość i jakość dostępnych w Google danych oraz informacji, łatwość ich wyszukiwania, liczba aktywnych użytkowników, a także częstotliwość, z jaką korzystają z wyszukiwarki sprawiają, że znaczenie Google w kreowaniu widoczności firm, produktów i usług w internecie jest bezdyskusyjna.
Jeśli nie realizujesz swojej strategii marketingowej w oparciu o wysokiej jakości usługi pozycjonowania stron, oznacza to, że nie dostrzegasz i być może marnujesz największy, obecnie dostępny potencjał marketingowo-sprzedażowy.
Liderzy o to dbają. Liderzy w to inwestują! Liderzy wierzą w pozycjonowanie.
Najczęściej zadawane pytania o pozycjonowanie stron
Na czym polega pozycjonowanie strony www?
Pozycjonowanie stron to działania marketingowe, których celem jest zdobycie i utrzymanie jak najwyższych pozycji dla określonych słów kluczowych w naturalnych wynikach wyszukiwania w wyszukiwarce internetowej. Na pozycjonowanie SEO składa się kilka kluczowych elementów, wśród których należy wskazać przede wszystkim: analizę sytuacji serwisu wraz z wykonaniem niezbędnych audytów, stworzenie strategii pozycjonowania w oparciu o cele biznesowe klienta, realizację działań związanych z optymalizacją techniczną, pozycjonowaniem on-site i off-site, wdrażanie zmian i rekomendacji oraz monitorowanie i analizę wyników.
Co wpływa na pozycjonowanie strony w Google?
Szczegółowy sposób działania algorytmu Google nie jest znany, ale istnieją pewne czynniki, które mają bezpośredni wpływ na pozycję, jaką zajmuje serwis w wynikach wyszukiwania na konkretne zapytanie. Wśród nich należy wyróżnić między innymi czynniki związane z: treściami na stronie (ich ilość, jakość, unikalność, nasycenie frazami kluczowymi, regularność publikacji, optymalizacja grafik, zdjęć, filmów, Core Web Vitals); profilem linkowym strony (jakość oraz liczba linków zewnętrznych); optymalizacją techniczną (prędkość ładowania strony, mapa witryny); domeną (historia domeny).
Jak sprawdzać efekty pozycjonowania strony?
Skuteczne pozycjonowanie strony www to takie, które realizuje cele biznesowe klienta. By zweryfikować, czy obrana strategia jest prawidłowa, należy regularnie weryfikować takie wskaźniki, jak: 1. widoczność – można ją łatwo sprawdzić w narzędziu Senuto w raporcie widoczności, który wskazuje liczbę fraz w TOP 3, TOP 10 i TOP organicznych wyników wyszukiwania; 2. wyświetlenia i kliknięcia – dane, które znajdują się w narzędziu Google Search Console, pozwalają zweryfikować, ile razy strona pojawiła się w wynikach wyszukiwania, a także ile razy użytkownicy kliknęli w wyświetlony link; 3. współczynnik CTR – to stosunek liczby kliknięć w link w wynikach organicznych w stosunku do liczby wyświetleń. Dane dotyczące współczynnika CTR również dostępne są w Google Search Console; 4. ruch organiczny – czyli pozyskany z naturalnych wyników wyszukiwania. Informacje na temat ruchu organicznego można znaleźć w Google Analytics; 5. konwersje – znajomość liczby, współczynnika i wartości konwersji pozwala na weryfikację, czy pozycjonowanie jest skuteczne, a realizacja przyjętej strategii SEO przełożyła się na wzrost przychodów.
Ile kosztuje pozycjonowanie strony?
Cena za pozycjonowanie stron SEO jest uzależniona od kilku kluczowych czynników: branży, w której funkcjonuje serwis, obecnej sytuacji witryny oraz wielkości konkurencji. Bez odpowiedniego budżetu możemy nie być w stanie świadczyć usługi pozycjonowania strony internetowej w taki sposób, by nadążyć zarówno za rynkiem, jak i rozwijającą się konkurencją.
Jak długo trwa pozycjonowanie w wyszukiwarce?
Efekty pozycjonowania mogą pojawić się zarówno po kilku tygodniach, jak i po kilku miesiącach od rozpoczęcia działań. To, ile trwa pozycjonowanie strony w Google, zależy przede wszystkim od branży, kompetencji specjalistów SEO, wdrożonej strategii, historii witryny oraz intensywności działań konkurencji.
Jak wypozycjonować stronę w krótkim czasie?
Pozycjonowanie stron internetowych bywa procesem długotrwałym – szczególnie że część prac, z uwagi na ich szeroki zakres, często jest realizowana etapami. Ponadto ze względu na zmiany w algorytmach wyszukiwarki, zachowaniu użytkowników czy działania konkurencji strategia pozycjonowania może wymagać aktualizacji czy modyfikacji. Jednak pierwsze efekty pozycjonowania i optymalizacji SEO mogą być widoczne już po kilku tygodniach.
Czy można pozycjonować stronę samodzielnie?
Samodzielne pozycjonowanie strony internetowej jest możliwe, jednak nie jest to proces łatwy, a nauka poszczególnych zagadnień wymaga sporo czasu. W takiej sytuacji warto poznawać krok po kroku kolejne zagadnienia, testować różne rozwiązania, a następnie monitorować i analizować efekty podejmowanych działań. Jeśli jednak oczekujesz możliwie szybkich efektów lub nie masz bądź możliwości, by zgłębiać tajniki pozycjonowania, najlepiej skorzystać z usług profesjonalnej agencji SEO.
Jak pozycjonować stronę za darmo?
Pozycjonowanie – czy to samodzielne, czy zlecone agencji SEO – zawsze wiąże się z inwestycją zarówno czasu, jak i środków, które trzeba przeznaczyć choćby na realizację treści, pozyskiwanie linków zewnętrznych czy dostęp do niektórych narzędzi. Jeśli chcesz zająć się tym we własnym zakresie, warto mieć świadomość, że realizacja skutecznej strategii pozycjonowania wymaga przy tym działań na wielu poziomach i w różnych obszarach, co nierzadko jest również bardzo czasochłonne. Decydując się natomiast na współpracę z profesjonalną agencją SEO, możesz skupić się przede wszystkim na swoim biznesie, a doświadczeni specjaliści pomogą Ci czerpać zyski płynące z większej widoczności Twojej firmy w sieci.
Co można zyskać dzięki pozycjonowaniu SEO?
Dzięki pozycjonowaniu stron www można zyskać przede wszystkim wzrost widoczności i poprawę pozycji na ważne dla Twojej firmy słowa kluczowe, zwiększenie wartościowego ruchu, a w rezultacie wzrost liczby zapytań i zwiększenie sprzedaży. Tym samym pozycjonowanie pozwala na zwiększenie rozpoznawalności Twojej marki w sieci, zainteresowania Twoimi produktami lub usługami i wreszcie – realizację założonych celów biznesowych.
Czy pozycjonowanie stron to usługa dla każdej firmy?
Pozycjonowanie to usługa, z której korzystają firmy z wielu branż – od sklepów internetowych, przez branże usługowe, po przemysł czy IT. Każda z nich ma własną specyfikę i mierzy się z różną konkurencją, jednak we wszystkich skuteczne pozycjonowanie stron www opierające się na prawidłowo dobranej strategii może przynieść liczne korzyści w postaci wzrostu widoczności, pozycji osiąganych na określone frazy kluczowe, a w efekcie zwiększenie wartościowego ruchu i sprzedaży.
Dlaczego pozycjonowanie wiąże się ze zmianami na stronie?
Proces pozycjonowania stron obejmuje między innymi optymalizację techniczną, rozbudowę istniejących treści i nasycenie ich frazami kluczowymi na poszczególnych podstronach czy tworzenie nowych tekstów. Tego typu działania wiążą się z koniecznością wdrażania zmian nie tylko w obszarze contentu, ale nierzadko też w strukturze menu, układzie czy kodzie strony. W razie braku możliwości wprowadzania zmian w obrębie serwisu, pozycjonowanie musiałoby ograniczać się wyłącznie do działań off-site, a tym samym mogłoby nie przynieść oczekiwanych rezultatów.
Skuteczne pozycjonowanie w Aktywnym Modelu Rozliczeń
Twoja agencja SEO działa standardowo? Doświadczasz stagnacji? To najwyższy czas, żeby sprawdzić możliwości Aktywnego Modelu Rozliczeń i postawić na skuteczne pozycjonowanie stron internetowych, ukierunkowane na potrzeby Twojego biznesu. Co cechuje Aktywny Model Rozliczeń?
BIZNES NA PIERWSZYM MIEJSCU
Na każdym etapie sprzedaży i realizacji rozmawiamy o Twoim biznesie. Poznajemy Twój biznes, a wykorzystując pozycjonowanie stron Google, realizujemy te cele, których najbardziej potrzebuje.
REALIZACJA BIZNESOWEGO CELU
Działania dopasowujemy do celu, a nie cel do oferowanych działań. Zapewniamy możliwość szybkiej zmiany tego, co robimy, aby osiągnąć wyznaczony cel.
WZROST BIZNESU
Kiedy Twój biznes się rozwija, my rozwijamy się razem z nim. Dlatego zależy nam na skalowaniu Twojego biznesu. Elastyczność pozwala na uwolnienie od barier i schematów narzuconych w abonamencie.
Pozycjonowanie stron Google ma nie tylko na celu zwiększenie widoczności strony i ruchu w serwisie. To przede wszystkim sposób na osiągnięcie tych celów, które mają największe znaczenie dla Twojego biznesu, a w rezultacie przełożą się na realne zyski. W abonamencie często brakuje pracy strategicznej i koncepcyjnej, ponieważ liczy się duża liczba wykonanych zadań prezentowanych w raporcie dla klienta. Możesz rozliczać agencję SEO z tych działań, ale równie dobrze możesz się skupić na wskaźnikach, które obrazują postępy w realizacji celu biznesowego. W Aktywnym Modelu Rozliczeń jest to możliwe. Sprawdź, jakie korzyści dla Twojego biznesu może przynieść Aktywny Model Rozliczeń. #wiemyjak
Pracuj ze zmotywowaną agencją!
Umów się na BEZPŁATNY BRIEF MARKETINGOWY. Zyskaj koncepcję skutecznego połączenia SEO z Twoim biznesem.
#wiemyjak
Skuteczne pozycjonowanie – co możesz zyskać z EACTIVE

- Wzrost widoczności na słowa kluczowe, które są ważne dla Twojego biznesu. Pozycjonowanie strony przełoży się na rozpoznawalność Twojej marki w sieci.
- Wzrost wartościowego ruchu, czyli przyciągnięcie użytkowników zainteresowanych oferowanymi przez Ciebie produktami, czy usługami.
- Wzrost liczby zapytań i zwiększenie sprzedaży, dzięki poprawie widoczności strony internetowej i osiąganych pozycji oraz pozyskaniu wartościowego ruchu.
- Holistyczne podejście do Twojego biznesu na każdym etapie sprzedaży i realizacji. Dzięki temu tworzymy strategię dopasowaną do oczekiwanych efektów i branży, w której funkcjonujesz, co przekłada się na skuteczne pozycjonowanie Twojej strony.
- Innowacyjne i elastyczne podejście do SEO. Szukamy innowacji w rozwiązaniach technicznych i programistycznych, dążąc do optymalnych i skutecznych działań w zakresie pozycjonowania.
- Pracę na celach. W Aktywnym Modelu Rozliczeń cel Twojego biznesu jest punktem, od którego zaczynamy planowanie działań pozycjonowania. Na etapie strategii określamy KPI oraz konkretne wskaźniki, które chcesz monitorować.
- Optymalne wykorzystanie budżetu. SEO wymaga inwestycji, dlatego dobieramy działania w taki sposób, aby zrealizować założone cele oraz utrzymać ROI i ROAS na określonym poziomie.
- Zespół specjalistów. Zbudowaliśmy profesjonalne zespoły specjalistów, którzy posiadają wiedzę i doświadczenie w obszarach technical SEO, SEO off-site, SEO content, analityki internetowej, UX oraz web development. Dzięki temu pozycjonowanie stron www realizujemy kompleksowo i na najwyższym poziomie.
- Przewagę nad konkurencją i ciągłe ulepszanie strony dla zwiększenia jej skuteczności. Koncentrujemy się nie tylko na pozycjonowaniu stron www, ale też przykładamy ogromną wagę do aspektów user experience, co pozwala zmienić w klientów tych użytkowników, którzy trafili na Twój serwis z bezpłatnych wyników wyszukiwania.
- Wiedzę i doświadczenie ekspertów. Wiemy, jak pozycjonować — jesteśmy nieprzerwanie od 10 lat w TOP 10 pod najtrudniejszą frazę w naszej branży: „pozycjonowanie stron”
Pracuj ze zmotywowaną agencją!
Umów się na BEZPŁATNY BRIEF MARKETINGOWY. Zyskaj koncepcję skutecznego połączenia SEO z Twoim biznesem.
#wiemyjak
Pozycjonowanie stron internetowych – jak działamy
Aby zbudować widoczność Twojej strony w sieci i w ten sposób pozyskać nowych klientów oraz wypracować wzrost konwersji, realizujemy pozycjonowanie stron internetowych – optymalizację SEO zarówno w szerokim, jak i szczegółowym zakresie, w skład którego wchodzą następujące elementy:

Audyt SEO

Strategia SEO

Technical SEO

Optymalizacja SEO

Link Building

Content marketing
Dla osiągnięcia najlepszych efektów pozycjonowania stron internetowych w trakcie współpracy zapewniamy opiekę dedykowanego zespołu specjalistów z zakresu SEO on-site, SEO off-site, content marketingu oraz analityki internetowej. Szczególną rolę w całym procesie odgrywają SEO Strategists, którzy wyznaczają kierunek działań podejmowanych przez zespół. Nad ich prawidłową realizacją oraz sprawną komunikacją z zespołem czuwać będzie natomiast Twój opiekun.
Dotrzyj do użytkowników wyszukujących usług i produktów w określonej lokalizacji. Realizujemy działania: pozycjonowanie Wrocław, pozycjonowanie Szczecin, pozycjonowanie Olsztyn, pozycjonowanie Białystok, pozycjonowanie Zielona Góra, pozycjonowanie Warszawa, pozycjonowanie Kraków, pozycjonowanie Łódź, pozycjonowanie Gdańsk, pozycjonowanie Poznań, pozycjonowanie Toruń, pozycjonowanie Lublin, pozycjonowanie Bydgoszcz, pozycjonowanie Kielce, pozycjonowanie Opole, pozycjonowanie Katowice, pozycjonowanie Rzeszów
Oczekujesz poważnego traktowania Twojego biznesu?
Napisz do nas, a skontaktujemy się z Tobą i przygotujemy propozycję współpracy.